Case Study 2: SARS-CoV-2 inference in The Gambia
David Hodgson
2025-12-03
cs2_sarscov2.RmdR Code Description
This document describes the steps and components of the provided R code for creating and running serological models using simulated data. The code defines observational models, antibody kinetics models, and runs inference using MCMC methods.
Step 2: Read Simulated Data
This data has been generated using serosim.
wave_full <- readRDS(file = "wave2_export.RDS")
#wave_full <- readRDS(file = "./vignettes/wave2_export.RDS")
sero_data_w2 <- wave_full$sero_data
known_inf_w2 <- wave_full$known_inf
inf_prior_w2 <- wave_full$inf_prior
# Check the entries are sensible
check_sero_no_single_entries(sero_data_w2)## No single entries in data_sero!,
check_sero_timings(sero_data_w2)## No individuals with less than 15 days in the study!
wave_full <- readRDS(file = "wave3_export.RDS")
#wave_full <- readRDS(file = "./vignettes/wave3_export.RDS")
sero_data_w3 <- wave_full$sero_data
known_inf_w3 <- wave_full$known_inf
inf_prior_w3 <- wave_full$inf_prior
# Check the entries are sensible
check_sero_no_single_entries(sero_data_w3)## No single entries in data_sero!,
check_sero_timings(sero_data_w3)## No individuals with less than 15 days in the study!
#known_inf_w2 <- known_inf_w2_check %>% filter(key_exposure == "inferrable")Step 3: Define Likelihood and Kinetics Functions
obsLogLikelihood <- function(titre_val, titre_est, pars) {
ll <- dnorm(titre_val, titre_est, pars[1], log = TRUE)
}
noInfSerumKinetics <- function(titre_est, timeSince, pars) {
titre_est_log <- titre_est - (pars[1] * titre_est) * (timeSince)
titre_est_log <- max(0, titre_est_log)
titre_est_log
}
infTuenisPower2016 <- function(titre_est, timeSince, pars) {
y1 <- pars[1]
t1 <- pars[2]
r <- pars[3]
alpha <- pars[4]
v <- 0.001
mu <- 1 / t1 * y1
if (timeSince < t1) {
titre_est_boost <- exp(mu * timeSince)
} else {
titre_est_boost <- exp(y1) * (1 + (r - 1) * exp(y1)^{r - 1} * v * (timeSince - t1)) ^ {-1 / (r - 1)}
}
titre_est_log <- titre_est + log(titre_est_boost) * max(0, 1 - titre_est * alpha)
titre_est_log
}Step 4: Define the Observational and Kinetics Models
# Define the biomarkers and exposure types in the model
biomarkers <- c("spike", "NCP")
exposureTypes <- c("none", "delta", "vax", "predelta")
exposureFitted <- "delta"
# Define the observational model
observationalModel <- list(
names = c("spike", "NCP"),
model = makeModel(
addObservationalModel("spike", c("sigma"), obsLogLikelihood),
addObservationalModel("NCP", c("sigma_a"), obsLogLikelihood)),
prior = bind_rows(
addPrior("sigma", 0.0001, 2, "exp", 1, NA),
addPrior("sigma_a", 0.0001, 2, "exp", 1, NA)
) # observational model,
)
# Define the antibody kinetics model
abkineticsModel <- list(
model = makeModel(
addAbkineticsModel("none_s", "spike", "none", c("wane"), noInfSerumKinetics),
addAbkineticsModel("delta_s", "spike", "delta", c("y1_d", "t1_d", "r_d", "s"), infTuenisPower2016),
addAbkineticsModel("vax_s", "spike", "vax", c("y1_vax", "t1_vax", "r_vax", "s"), infTuenisPower2016),
addAbkineticsModel("predelta_s", "spike", "predelta", c("y1_pd", "t1_pd", "r_pd", "s"), infTuenisPower2016),
addAbkineticsModel("none_ncp", "NCP", "none", c("wane_a"), noInfSerumKinetics),
addAbkineticsModel("delta_ncp", "NCP", "delta", c("y1_d_a", "t1_d_a", "r_d_a", "s_a"), infTuenisPower2016),
addAbkineticsModel("vax_ncp", "NCP", "vax", c("y1_vax_a", "t1_vax_a", "r_vax_a", "s_a"), infTuenisPower2016),
addAbkineticsModel("predelta_ncp", "NCP", "predelta", c("y1_pd_a", "t1_pd_a", "r_pd_a", "s_a"), infTuenisPower2016)
),
prior = bind_rows(
addPrior("y1_d", 0, 6, "unif", 0, 6), # ab kinetics
addPrior("t1_d", 7, 21, "unif", 7, 21), # ab kinetics
addPrior("r_d", 1, 5, "unif", 1, 5), # ab kinetics
addPrior("y1_vax", 0, 6, "unif", 0, 6), # ab kinetics
addPrior("t1_vax", 7, 21, "unif", 7, 21), # ab kinetics
addPrior("r_vax", 1, 5, "unif", 1, 5), # ab kinetics
addPrior("y1_pd", 0, 6, "unif", 0, 6), # ab kinetics
addPrior("t1_pd", 7, 21, "unif", 7, 21), # ab kinetics
addPrior("r_pd", 1, 5, "unif", 1, 5), # ab kinetics
addPrior("s", 0, 1, "unif", 0, 1), # ab kinetics
addPrior("wane", 0.0, 0.01, "unif", 0.0, 0.01), # observational model
addPrior("y1_d_a", 0, 6, "unif", 0, 6), # ab kinetics
addPrior("t1_d_a", 7, 21, "unif", 7, 21), # ab kinetics
addPrior("r_d_a", 1, 5, "unif", 1, 5), # ab kinetics
addPrior("y1_vax_a", 0, 6, "unif", 0, 6), # ab kinetics
addPrior("t1_vax_a", 7, 21, "unif", 7, 21), # ab kinetics
addPrior("r_vax_a", 1, 5, "unif", 1, 5), # ab kinetics
addPrior("y1_pd_a", 0, 6, "unif", 0, 6), # ab kinetics
addPrior("t1_pd_a", 7, 21, "unif", 7, 21), # ab kinetics
addPrior("r_pd_a", 1, 5, "unif", 1, 5), # ab kinetics
addPrior("s_a", 0, 1, "unif", 0, 1), # ab kinetics
addPrior("wane_a", 0.0, 0.01, "unif", 0.0, 0.01) # observational model
)
)
model_w2 <- createSeroJumpModel(
data_sero = sero_data_w2,
data_known = known_inf_w2,
biomarkers = biomarkers,
exposureTypes = exposureTypes,
exposureFitted = exposureFitted,
observationalModel = observationalModel,
abkineticsModel = abkineticsModel,
exposurePriorTime = inf_prior_w2,
exposurePriorTimeType = "empirical"
)## OUTLINE OF INPUTTED MODEL
## There are 2 measured biomarkers: spike, NCP
## There are 4 exposure types in the study period: none, delta, vax, predelta
## The fitted exposure type is delta
## PRIOR DISTRIBUTIONS
## Prior parameters of observationalModel are: sigma, sigma_a
## Prior parameters of abkineticsModel are: y1_d, t1_d, r_d, y1_vax, t1_vax, r_vax, y1_pd, t1_pd, r_pd, s, wane, y1_d_a, t1_d_a, r_d_a, y1_vax_a, t1_vax_a, r_vax_a, y1_pd_a, t1_pd_a, r_pd_a, s_a, wane_a
## No single entries in data_sero!,
## No individuals with less than 15 days in the study!## Warning in addExposurePrior_checkempirical(exp_prior, T_max): Warning: The number of rows in the exposure prior dataframe does not match the number of time points in the study
## Warning in addExposurePrior_checkempirical(exp_prior, T_max): Warning: The number of rows in the exposure prior dataframe does not match the number of time points in the study## There are individuals which have exposure before their first bleed or before their last bleed, ids: 19, 23, 45, 92, 95, 147, 162, 205, 218, 6, 18, 24, 30, 63, 98, 142, 181, 192, 199, 208, 210, 214, 215, 245, 246, 254, 265 . It is hard to perform inference on these people, so we recommend you remove the exposures from the known inf, but, if included, the model ignores these exposures.Step 4B: Prerun sanity plots
Before running the whole model it is good to check the data and the
priors. This can be done using a suit of functions
plotPriors function.
p1 <- plotSero(model_w2)
p2 <- plotPriorPredictive(model_w2)## Warning: Using `size` aesthetic for lines was deprecated in ggplot2 3.4.0.
## ℹ Please use `linewidth` instead.
## ℹ The deprecated feature was likely used in the serojump package.
## Please report the issue to the authors.
## This warning is displayed once every 8 hours.
## Call `lifecycle::last_lifecycle_warnings()` to see where this warning was
## generated.
p3 <- plotPriorInfection(model_w2)
p1 / p2## Ignoring unknown labels:
## • colour : "Exposure type"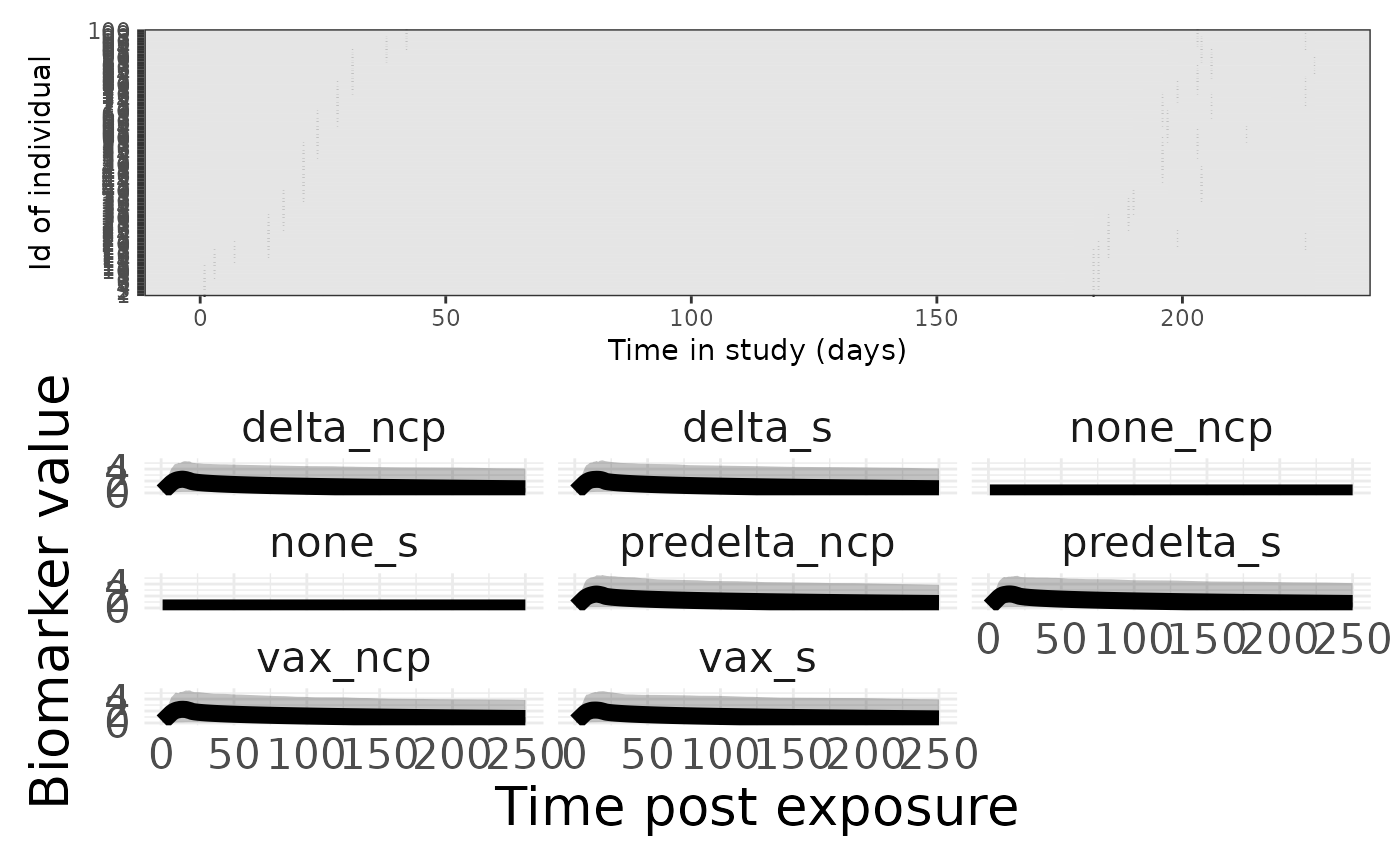
p3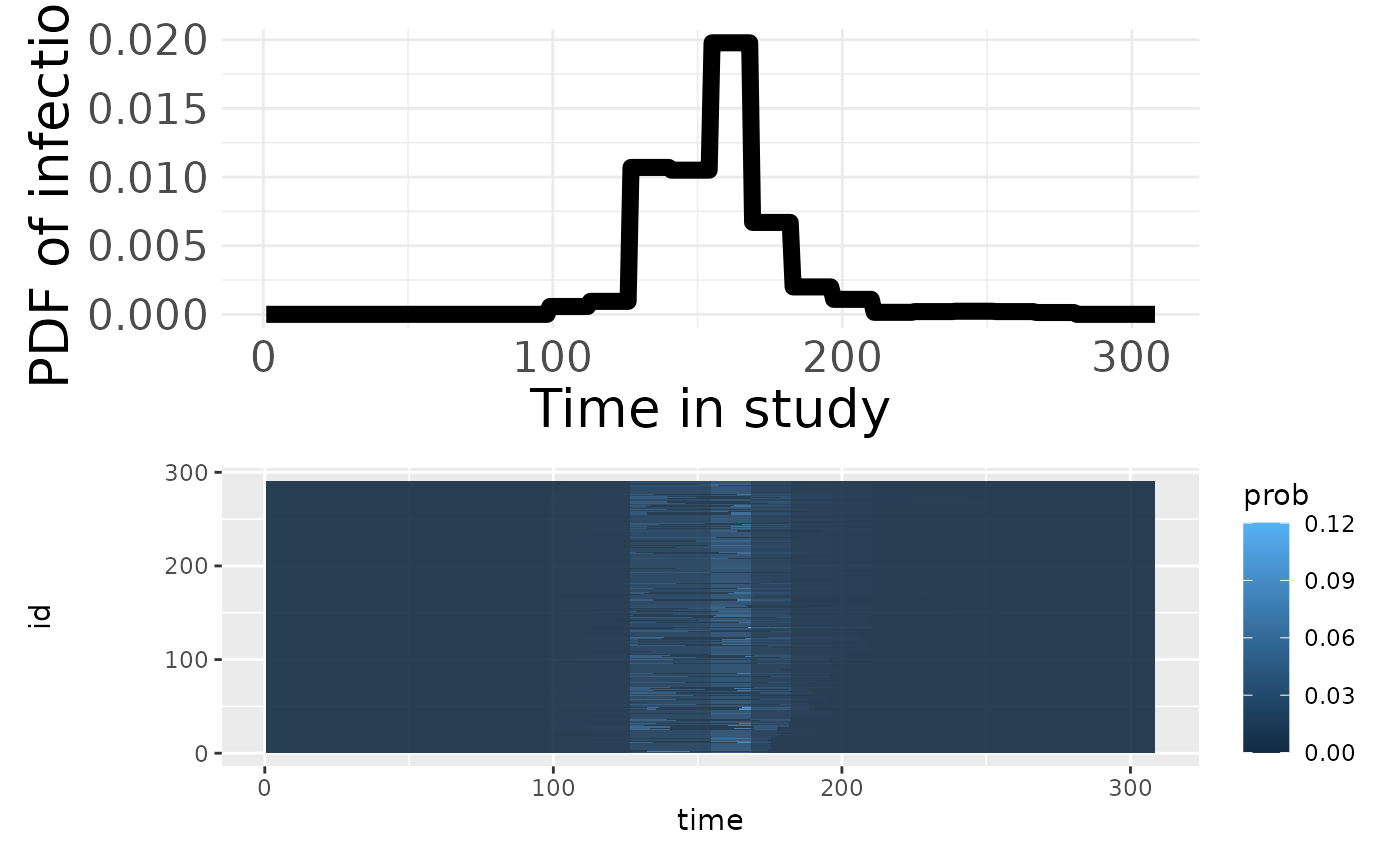
Step 5: Define the Complete Model
# Define the biomarkers and exposure types in the model
biomarkers <- c("spike", "NCP")
exposureTypes <- c("none", "omicron", "vax")
exposureFitted <- "omicron"
# Define the observational model
observationalModel <- list(
names = c("spike", "NCP"),
model = makeModel(
addObservationalModel("spike", c("sigma"), obsLogLikelihood),
addObservationalModel("NCP", c("sigma_a"), obsLogLikelihood)),
prior = bind_rows(
addPrior("sigma", 0.0001, 2, "exp", 1, NA),
addPrior("sigma_a", 0.0001, 2, "exp", 1, NA)
) # observational model,
)
# Define the antibody kinetics model
abkineticsModel <- list(
model = makeModel(
addAbkineticsModel("none_s", "spike", "none", c("wane"), noInfSerumKinetics),
addAbkineticsModel("omicron_s", "spike", "omicron", c("y1_o", "t1_o", "r_o", "s"), infTuenisPower2016),
addAbkineticsModel("vax_s", "spike", "vax", c("y1_vax", "t1_vax", "r_vax", "s"), infTuenisPower2016),
addAbkineticsModel("none_ncp", "NCP", "none", c("wane_a"), noInfSerumKinetics),
addAbkineticsModel("omicron_ncp", "NCP", "omicron", c("y1_o_a", "t1_o_a", "r_o_a", "s_a"), infTuenisPower2016),
addAbkineticsModel("vax_ncp", "NCP", "vax", c("y1_vax_a", "t1_vax_a", "r_vax_a", "s_a"), infTuenisPower2016)
),
prior = bind_rows(
addPrior("y1_o", 0, 6, "unif", 0, 6), # ab kinetics
addPrior("t1_o", 7, 21,"unif", 7, 21), # ab kinetics
addPrior("r_o", 1, 5, "unif", 1, 5), # ab kinetics
addPrior("y1_vax", 0, 6, "unif", 0, 6), # ab kinetics
addPrior("t1_vax", 7, 21, "unif", 7, 21), # ab kinetics
addPrior("r_vax", 1, 5, "unif", 1, 5), # ab kinetics
addPrior("s", 0, 1, "unif", 0, 1), # ab kinetics
addPrior("wane", 0.0, 0.01, "unif", 0.0, 0.01), # observational model
addPrior("y1_o_a", 0, 6, "unif", 0, 6), # ab kinetics
addPrior("t1_o_a", 7, 21, "unif", 7, 21), # ab kinetics
addPrior("r_o_a", 1, 5, "unif", 1, 5), # ab kinetics
addPrior("y1_vax_a", 0, 6, "unif", 0, 6), # ab kinetics
addPrior("t1_vax_a", 7, 21, "unif", 7, 21), # ab kinetics
addPrior("r_vax_a", 1, 5, "unif", 1, 5), # ab kinetics
addPrior("s_a", 0, 1, "unif", 0, 1), # ab kinetics
addPrior("wane_a", 0.0, 0.01, "unif", 0.0, 0.01) # observational model
)
)
model_w3 <- createSeroJumpModel(
data_sero = sero_data_w3,
data_known = known_inf_w3,
biomarkers = biomarkers,
exposureTypes = exposureTypes,
exposureFitted = exposureFitted,
observationalModel = observationalModel,
abkineticsModel = abkineticsModel,
exposurePriorTime = inf_prior_w3,
exposurePriorTimeType = "empirical"
)## OUTLINE OF INPUTTED MODEL
## There are 2 measured biomarkers: spike, NCP
## There are 3 exposure types in the study period: none, omicron, vax
## The fitted exposure type is omicron
## PRIOR DISTRIBUTIONS
## Prior parameters of observationalModel are: sigma, sigma_a
## Prior parameters of abkineticsModel are: y1_o, t1_o, r_o, y1_vax, t1_vax, r_vax, s, wane, y1_o_a, t1_o_a, r_o_a, y1_vax_a, t1_vax_a, r_vax_a, s_a, wane_a
## No single entries in data_sero!,
## No individuals with less than 15 days in the study!## Warning in addExposurePrior_checkempirical(exp_prior, T_max): Warning: The number of rows in the exposure prior dataframe does not match the number of time points in the study
## Warning in addExposurePrior_checkempirical(exp_prior, T_max): Warning: The number of rows in the exposure prior dataframe does not match the number of time points in the study## There are individuals which have exposure before their first bleed or before their last bleed, ids: 111, 57, 227, 228, 248 . It is hard to perform inference on these people, so we recommend you remove the exposures from the known inf, but, if included, the model ignores these exposures.Step 5B: Prerun sanity plots
Before running the whole model it is good to check the data and the
priors. This can be done using a suit of functions
plotPriors function.
p1 <- plotSero(model_w3)
p2 <- plotPriorPredictive(model_w3)
p3 <- plotPriorInfection(model_w3)
p1 / p2## Ignoring unknown labels:
## • colour : "Exposure type"
p3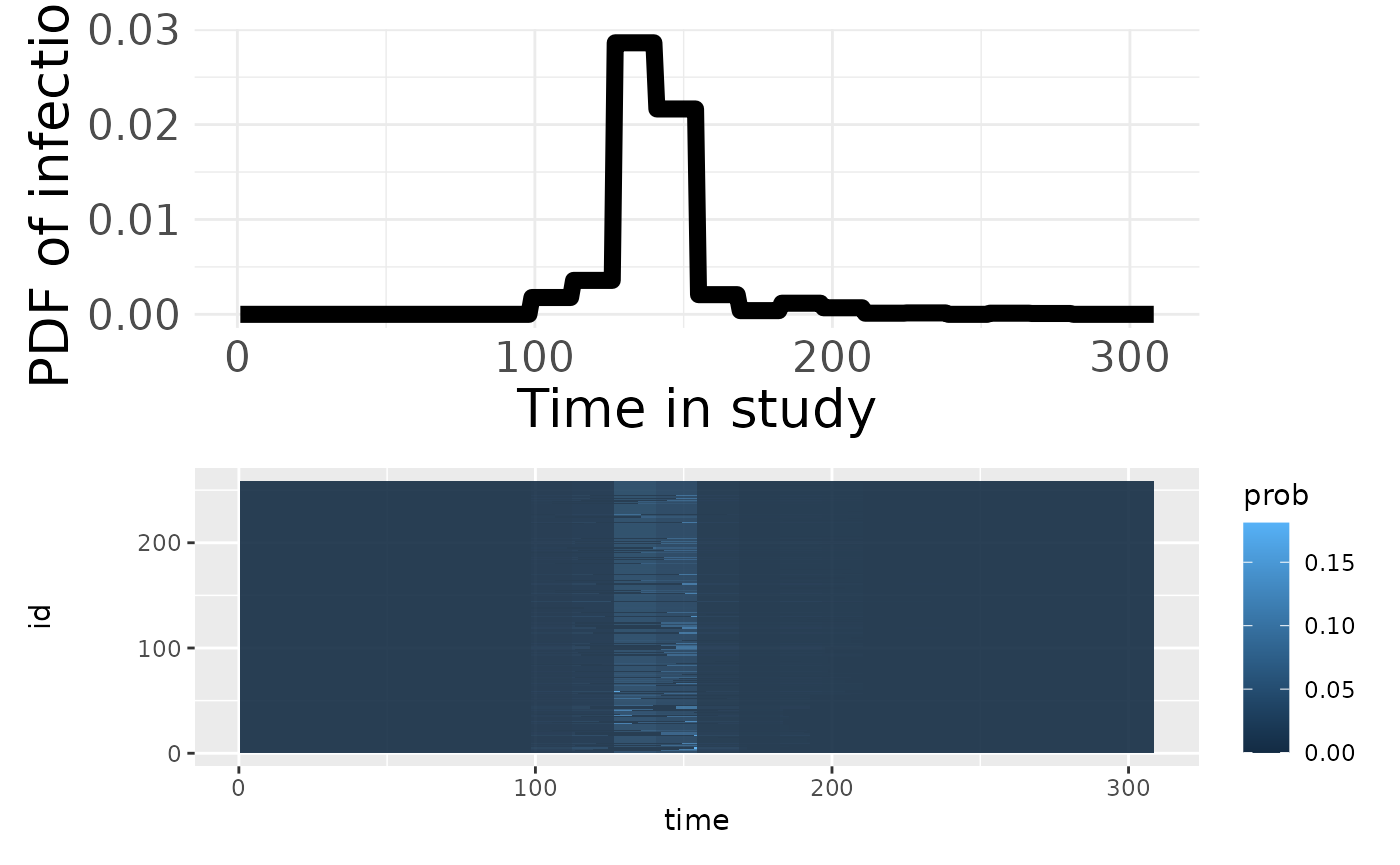
rj_settings <- list(
numberChainRuns = 4,
iterations = 200000,
burninPosterior = 100000,
thin = 100
# onDebug = TRUE,
# runParallel = FALSE
)
save_info_w2 <- list(
file_name = "transvir_data",
model_name = "wave2_base"
)
save_info_w3 <- list(
file_name = "transvir_data",
model_name = "wave3_base"
)
# Run these but take a while
#model_summary_w2 <- runSeroJump(model_w2, rj_settings, save_info = save_info_w2)
#model_summary_w3 <- runSeroJump(model_w3, rj_settings, save_info = save_info_w3)
model_summary_w2 <- readRDS("model_summary_w2.RDS")
model_summary_w3 <- readRDS("model_summary_w3.RDS")
plotMCMCDiagnosis(model_summary_w2, save_info = save_info_w2)
# takes ages to run these so comment out!
#plotPostFigs(model_summary_w2, save_info = save_info_w2)
plotMCMCDiagnosis(model_summary_w3, save_info = save_info_w3)
# takes ages to run these so comment out!
#plotPostFigs(model_summary_w3, save_info = save_info_w3)